Investigating How Cells Recognize Good and Bad Fats
Virginia McDonough-Stukey, Ph.D. | Professor of Biology
We may have to check the nutrition label to know the amounts of saturated and unsaturated fats we’re consuming, but for our cells, this tallying is second nature. Scientists have known for years that cells absorb, process and use fats; that they can change saturated fats to unsaturated ones; and that they recognize the difference between the two. What remains a mystery is just how they make that distinction.
That’s what molecular biologist Dr. Virginia McDonough, a professor and researcher at Hope since 1995, wants to find out. She and her students have been studying the way that cells recognize and respond to the presence of saturated fats (commonly called “bad” fats) and unsaturated (“good”) fats. McDonough’s research group is tantalizingly close to understanding our cells’ approach to doing this and has identified some key components of that process.
“We have found quite a few of the proteins that work as part of the system,” McDonough says. “We know a lot of the pieces; we just haven’t put the pieces together into the puzzle yet.” A three-year, $246,972 National Institutes of Health grant awarded in 2019 funds the current phase of her long-term research.
“I think people recognize that we’re really close to being able to put those pieces together,” McDonough adds. “I’m hoping that in the next couple of years we’re going to be able to come up with some very nice models of how the system works.”
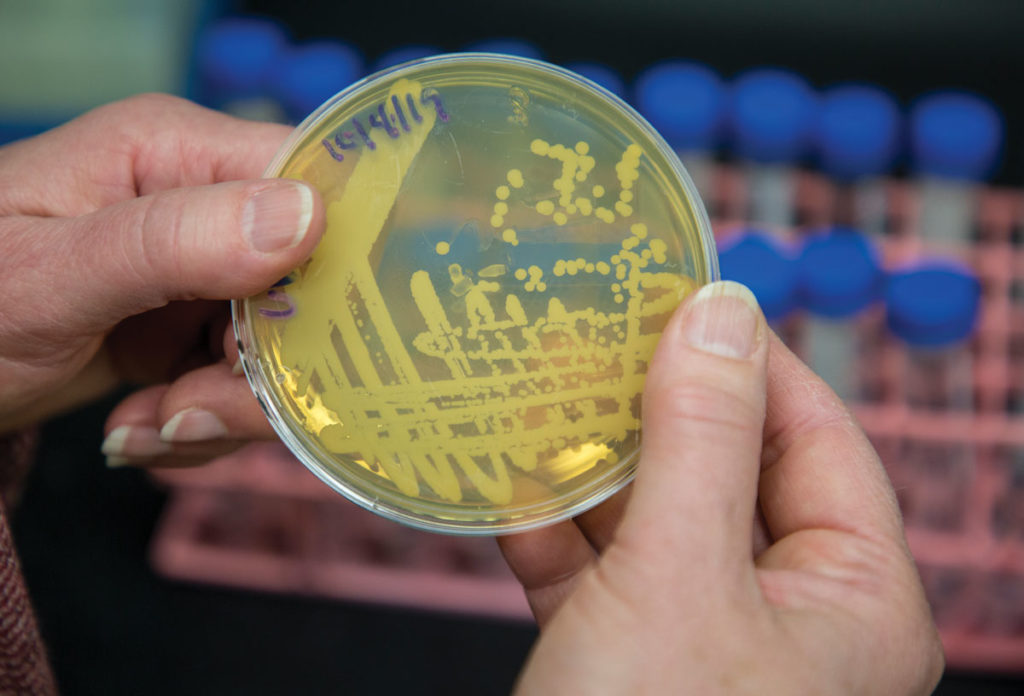
Models — specifically model organisms — have already provided a substantial source of insight for McDonough’s lab (and for science more broadly). By mutating select genes in yeast — a common model organism — McDonough and her team disrupt the ability of the cell to produce some specific proteins.
This makes that pathway dysfunctional and impairs the cell’s ability to metabolize fat. Once McDonough identified some of the proteins involved in the fat sensing/regulating system, the next step was to disrupt them through this selective gene mutation and observe the results.
“We’re trying to study how the system should work normally,” she says. “We do that by probing it when it’s not working normally, and then figure out what’s gone wrong.”
When fat metabolism goes wrong in human cells, a whole host of disorders can follow. Obesity, diabetes, cardiovascular disease and certain cancers are all linked to dysfunctional fat metabolism.
One of the things McDonough knows throws off cellular fat metabolism is a glitch in the so-called “chemical switches” that regulate the production of an enzyme — known in biochemistry circles as stearoyl-CoA desaturase — that converts saturated fats into unsaturated ones. When functioning properly, the chemical switch (or switches, as the case may be) turns off production of the enzyme when the cell has enough unsaturated fat.
McDonough likens the process to a bucket brigade: “There are proteins that are recognizing something that has to do with the diet, and they’re telling other proteins, ‘There’s plenty of unsaturated fats; you should shut down expression of the desaturase — we don’t need it.’” Conversely, a second switch could tell other proteins to restart the desaturase expression when unsaturated fat levels drop. The switches, working in alternate shifts, keep the cell’s lipid (fat) levels optimally balanced.
“We know that [the chemical switches are] there, because we can see the effect,” she says. “We just don’t know what they are or how they work.” To narrow the search, she and the Hope students in her research group are targeting the beginning of the desaturation process. “I’m pretty confident that there are switches early in the process, based on some of the previous work that we’ve done,” McDonough says. “So we should be able to find them.” Once found, these switches can be plugged into the lab’s burgeoning model of how the desaturation process, and the regulation of that process, work. The puzzle will be one piece closer to its resolution.
McDonough is occasionally asked, “Why not just study cancer? Why focus on the brass tacks way down at the cellular level?” Part of the answer is that McDonough has always been attracted by the intricacies of lipid metabolism. “I’ve always liked lipids. They’re sort of the neglected corner, but I’ve always found them really interesting,” she says.
A bigger part of the answer is that much of what affects cancer cells — and, for that matter, patients affected by diabetes, or cardiovascular disease, or obesity — is still unknown. “Early on, nobody thought to look at glucose metabolism,” she says, “but once scientists did, we understood how these abnormal cells get their energy to keep dividing. That may give us a clue as to how to treat this disease. You can’t just be focused on translational things; you still have to continue the work on basic research.”
McDonough also focuses on training others in that work. “I like people to know how important it is for students to have the opportunity to do research, because this is the next generation of scientists, and they need to get their training started — the sooner the better,” she says. “Meanwhile, I feel like it’s really important for us to continue basic research that we do here, so that we understand how things work — because that lets us figure out why they’re broken.”